
CE-IVD and NMPA Approved | Precision Molecular Diagnostics for Solid Tumors
Genecast Focus is a clinically validated, CE-IVD and NMPA approved targeted NGS assay for solid tumor profiling. It enables accurate detection of key biomarkers across 41 driver genes and MSI status, empowering personalized oncology treatment decisions
DNA extracted from FFPE tissue is used to detect SNVs, Indels, CNVs, and MSI, while RNA is analyzed to identify gene fusions and rearrangements. The kit accurately and rapidly provides patients with mutation information relevant to targeted drugs, immunotherapy drugs .
Guiding Treatment with Actionable Genomic Insights
Precise matching for targeted therapy:
Simplifies the screening process for targeted therapy and accelerates clinical decision-making. By detecting mutations, amplifications, or fusions in common driver genes (e.g., EGFR, KRAS, BRAF, ALK, ROS1), the test helps clinicians determine whether a patient is suitable for targeted treatment.
Identification of potential beneficiaries of immunotherapy:
Supports assessment of immunotherapy indications, particularly for patients with MSI-H or mutations in POLE/POLD1.
Multi-Dimensional Gene List Aligned with Clinical Needs
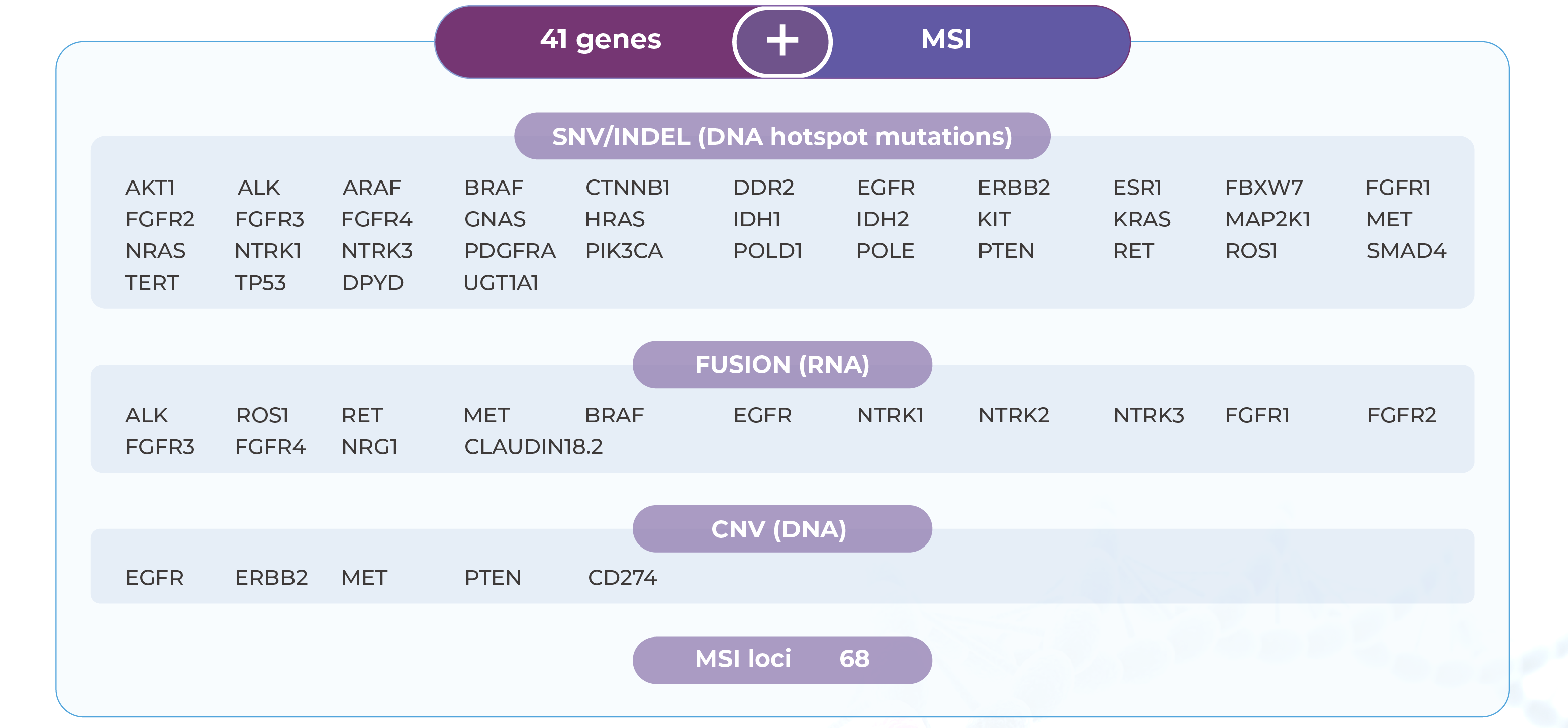
Applicable Across a Wide Range of Solid Tumors
Targeting 41 Driver Genes and MSI to Support Personalized Treatment Decisions
Suitable for non-small cell lung cancer, colorectal cancer, gastric cancer, gastrointestinal stromal tumors, thyroid cancer, bladder cancer, esophageal cancer, melanoma, biliary tract malignancies, and a wide range of other solid tumors.
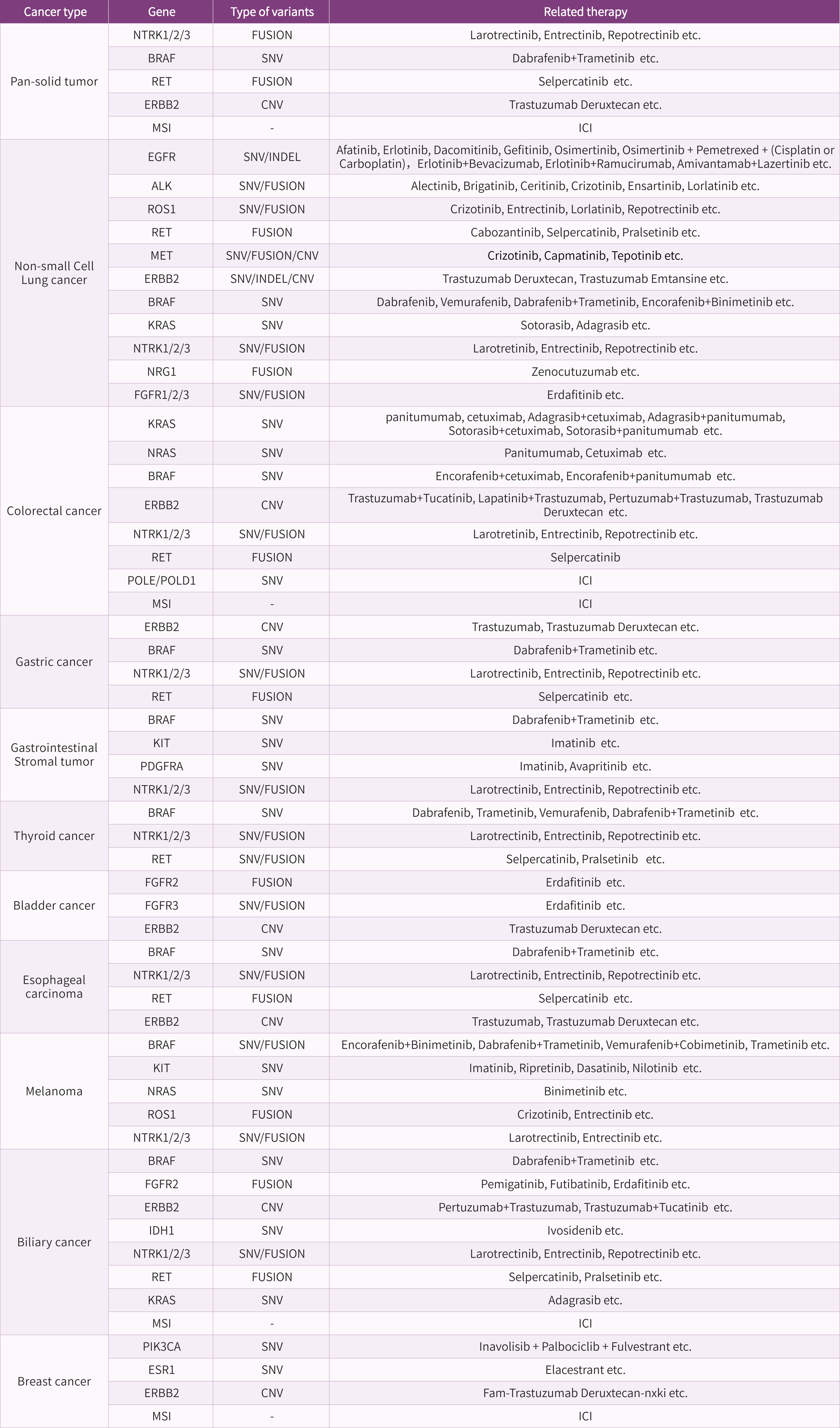
Who Should Consider This Test?
Patients with solid tumors requiring initial treatment decision support
To assess eligibility for targeted therapies and potential benefit from immunotherapy based on key biomarkers such as EGFR, ALK, BRAF mutations, MSI-H, and POLE/POLD1 mutations.
Patients seeking more comprehensive profiling through combined DNA and RNA analysis
Suitable for identifying both gene mutations and gene fusions. Provides a more complete molecular landscape to support personalized treatment decisions
Patients with limited or low-quality tumor tissue samples
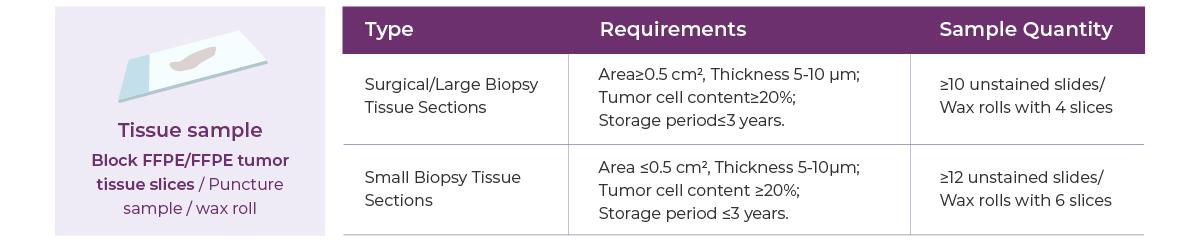
Small biopsy volume or archived FFPE samples collected within the past 3 years
Patients in early-stage or locally advanced settings
To support neoadjuvant treatment planning and post-surgical risk stratification and adjuvant therapy selection
Why Choose Genecast Focus
Comprehensive Coverage of Actionable Genes
Genecast Focus targets 41 key driver genes and over 900 hotspot regions across major solid tumors such as non-small cell lung cancer and colorectal cancer. It provides clinically actionable information to guide both targeted therapies and immunotherapy decisions.
Highly Sensitive Detection with Minimal Input
With as little as 10 ng of DNA, the assay can detect somatic mutations down to 1% allele frequency. Using just 50 ng of RNA, it reliably identifies gene fusions and rearrangements with a detection limit of 100 copies, making it suitable even for low-yield clinical samples.
Flexible Compatibility with Challenging Samples
Focus is optimized for formalin-fixed, paraffin-embedded (FFPE) tissue samples, including those with degraded DNA or RNA. This makes it ideal for use with archived or limited-quantity tumor specimens.
Internationally Certified and Clinically Approved
Genecast Focus is CE-IVD certified and approved by the NMPA in China, ensuring high standards of quality, reliability, and regulatory compliance for clinical application.
Sample Requirements
Testing Workflow & Ordering Process
TAT time:2 bussiness days*
(*) Turn-around time takes effect upon sample arrival at Genecast Singapore lab and clears sample quality checks.
Complete the Test Requisition Form
The ordering physician or patient fills out the form with relevant clinical and sample information.
Send Samples
Submit the required samples—such as FFPE blocks/slides/wax roll—to the Genecast laboratory.
Sample Receipt & Registration
Samples are inspected upon arrival and logged into our system for processing.
DNA & RNA Extraction
High-quality DNA and RNA are extracted from the submitted samples.
Library Preparation
Sequencing libraries are prepared using standardized protocols to ensure accuracy and consistency.
Next-Generation Sequencing
High-throughput sequencing is performed to detect relevant genomic alterations.
Bioinformatics Analysis
Advanced proprietary algorithms are applied to identify clinically actionable variants.
Report Delivery
The test report is generated and delivered to the designated email for clinical use.