
Comprehensive NGS Solution for Therapy Selection and Treatment Monitoring Across Solid Tumors
Applicable Across Multiple Solid Tumor Types
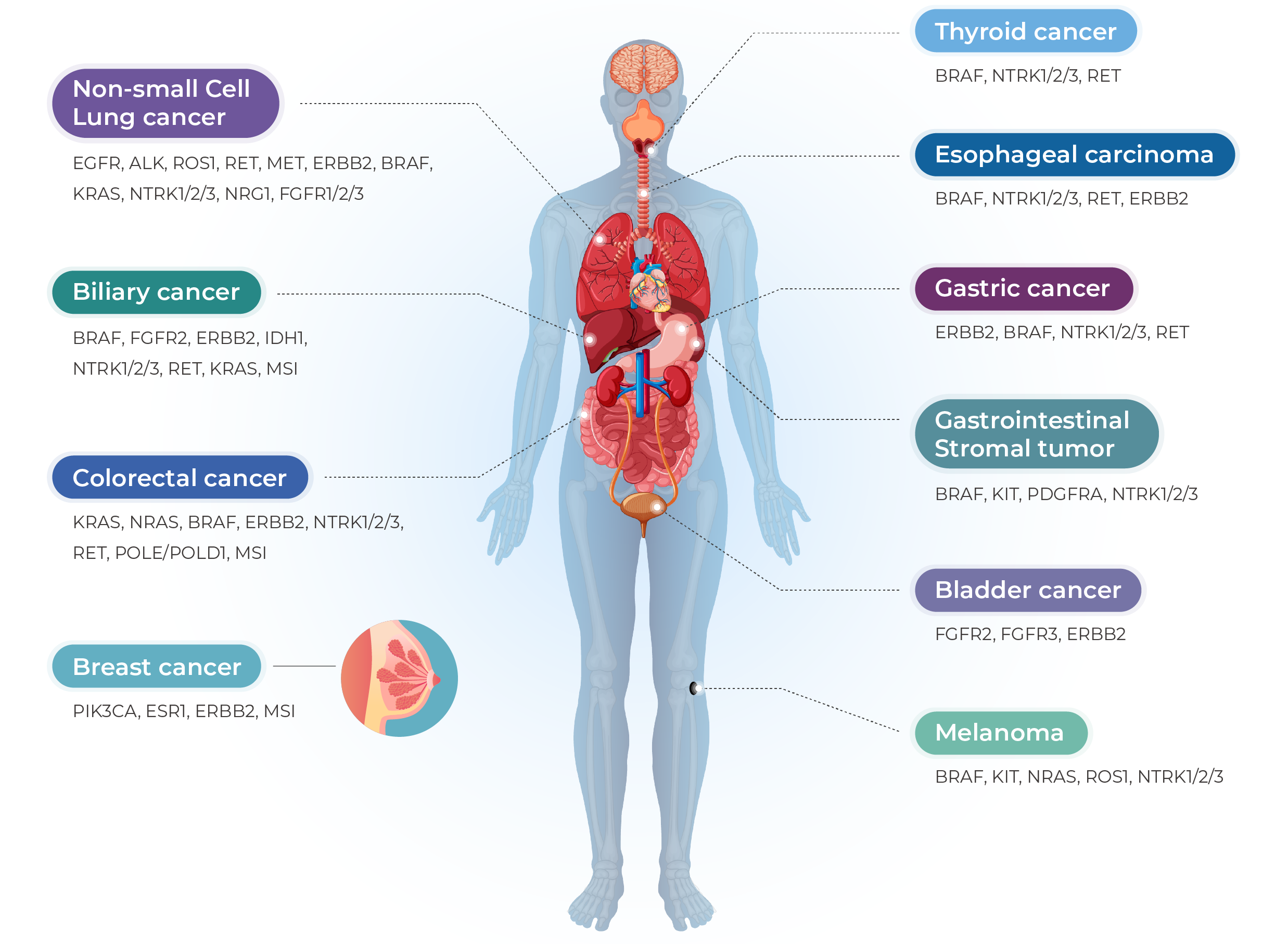
Lung cancer, colorectal cancer, breast cancer, gastric cancer, gastrointestinal stromal tumor (GIST), thyroid cancer, head and neck squamous cell carcinoma, ovarian cancer, melanoma, and other solid tumors.
Enabling Precision Treatment Decisions Across the Cancer Care Continuum
Support both adjuvant and advanced systemic therapy decision-making across major tumor types by identifying clinically actionable mutations from FFPE samples.
Evaluate eligibility for targeted and immunotherapies through actionable genomic alterations and key biomarkers such as TMB, MSI indicators.
Uncover resistance mechanisms by detecting secondary mutations post-treatment, enabling timely adjustment of therapy lines.
Provide molecular eligibility for clinical trial enrollment and off-label targeted therapies based on guideline-recommended biomarkers.
Molecular Profiling Redefines Cancer Treatment
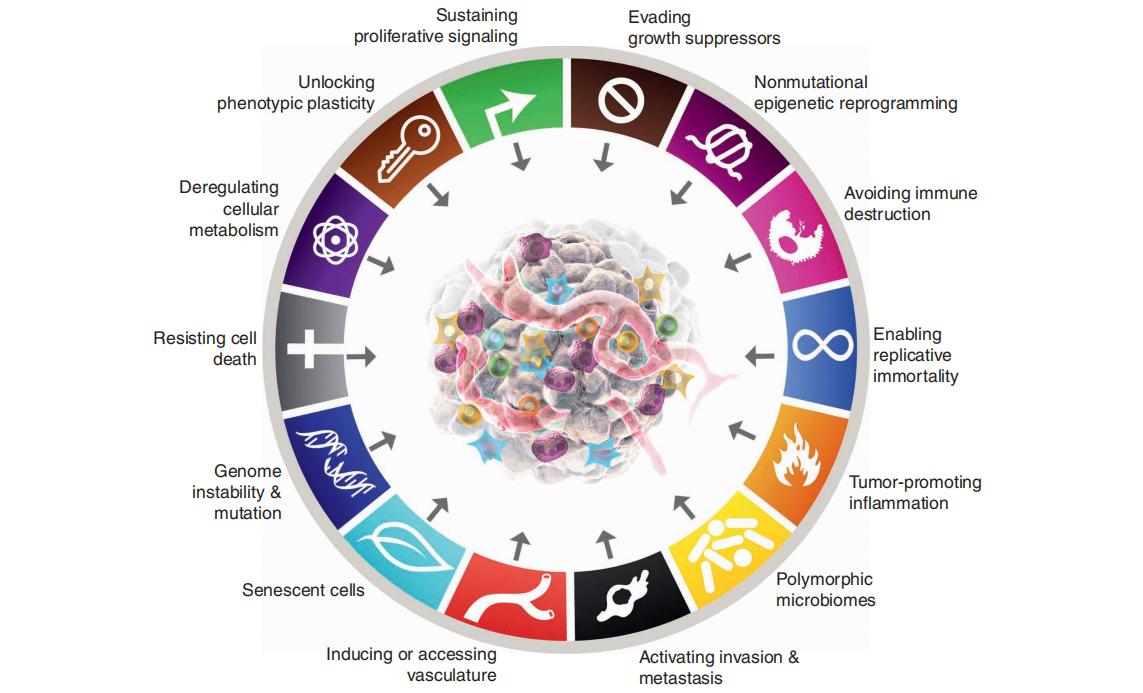
Researchers have found that the characteristics of tumors are determined by their genetic makeup. Therefore, cancer treatment can begin at the genetic level by targeting specific genes to influence tumor behavior and achieve therapeutic outcomes. Tumor driver genes are associated with cancer progression and can help assess prognosis, as well as the risks of recurrence and metastasis. Drug-related genes provide valuable guidance for therapy selection by indicating drug sensitivity or resistance. Understanding the genetic profile of a tumor forms the basis for treatment planning. By identifying the appropriate genes, more precise and effective cancer therapies can be delivered, maximizing clinical benefit.
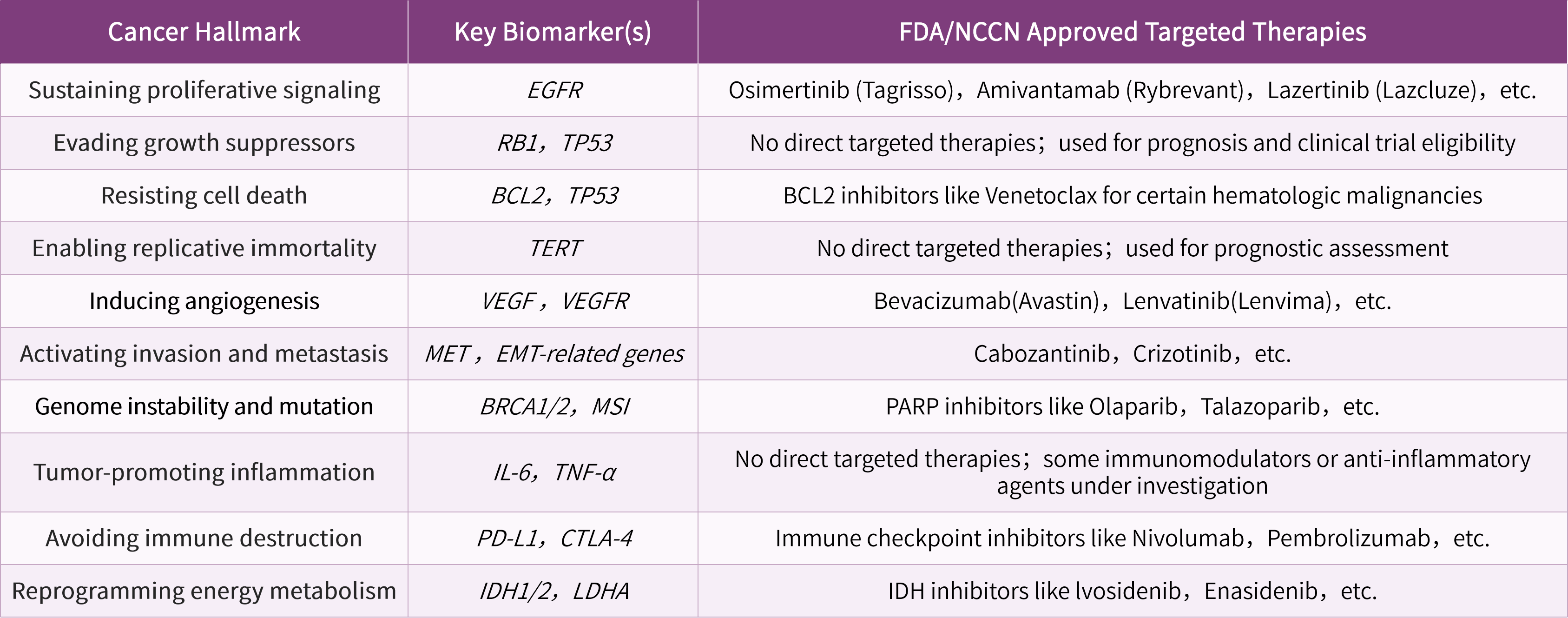
Cancer Discov. 2022 Jan;12(1):31-46.
Multiple studies have shown that NGS-based molecular profiling in patients with advanced or metastatic solid tumors can identify potentially actionable genetic alterations in approximately 80% of cases. Among those who received molecularly guided therapy, more than one-third experienced a clear clinical benefit, with a subset achieving durable disease control for 12 months or longer. These findings highlight the value of molecular profiling in enabling personalized treatment strategies that can significantly improve therapeutic outcomes and survival expectations.
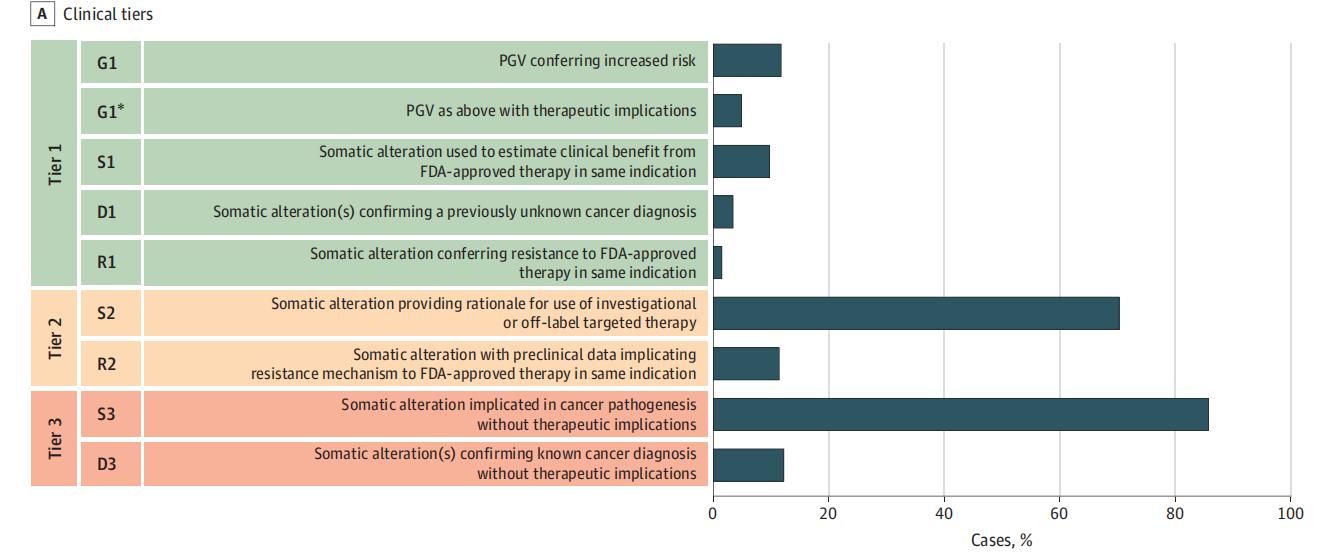
JAMA Oncol. 2021 Apr 1;7(4):525-533.
In the past two decades, cancer has been gradually redefined.
How Does Genecast Comprehensive Support Clinical Decision-Making?
Supports Adjuvant and Systemic Therapy Decisions
Detects SNVs, CNVs, and InDels across 769 genes at the DNA level, and 52 clinically relevant fusions at the RNA level, enabling personalized treatment planning for both adjuvant and systemic settings.
Guides Immunotherapy Suitability Assessment
Reports TMB, MSI, and POLE/POLD1 mutation status to evaluate the likelihood of benefit from immune checkpoint inhibitors.
Uncovers Resistance Mechanisms to Guide Subsequent Therapy
Identifies key resistance mutations (e.g., EGFR T790M, KRAS G12C, HER2 amplification), providing molecular insights for optimizing second- and third-line treatment strategies.
Assesses Recurrence Risk and Potential Residual Disease
Detects high-risk mutations such as TP53, PIK3CA, and RB1 that may be associated with minimal residual disease or postoperative recurrence, supporting long-term follow-up planning.
Facilitates Clinical Trial Matching
Covers multiple NCCN-endorsed biomarkers to assist in matching patients to targeted therapy or immunotherapy trials.
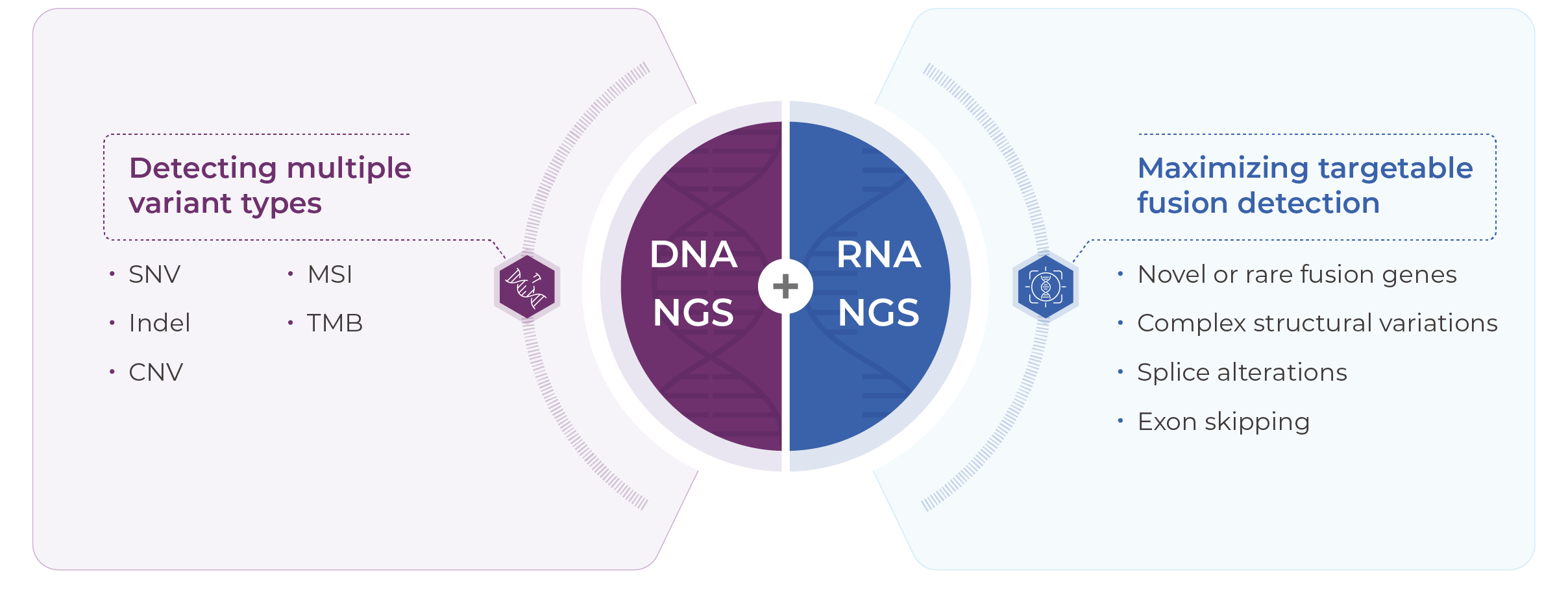
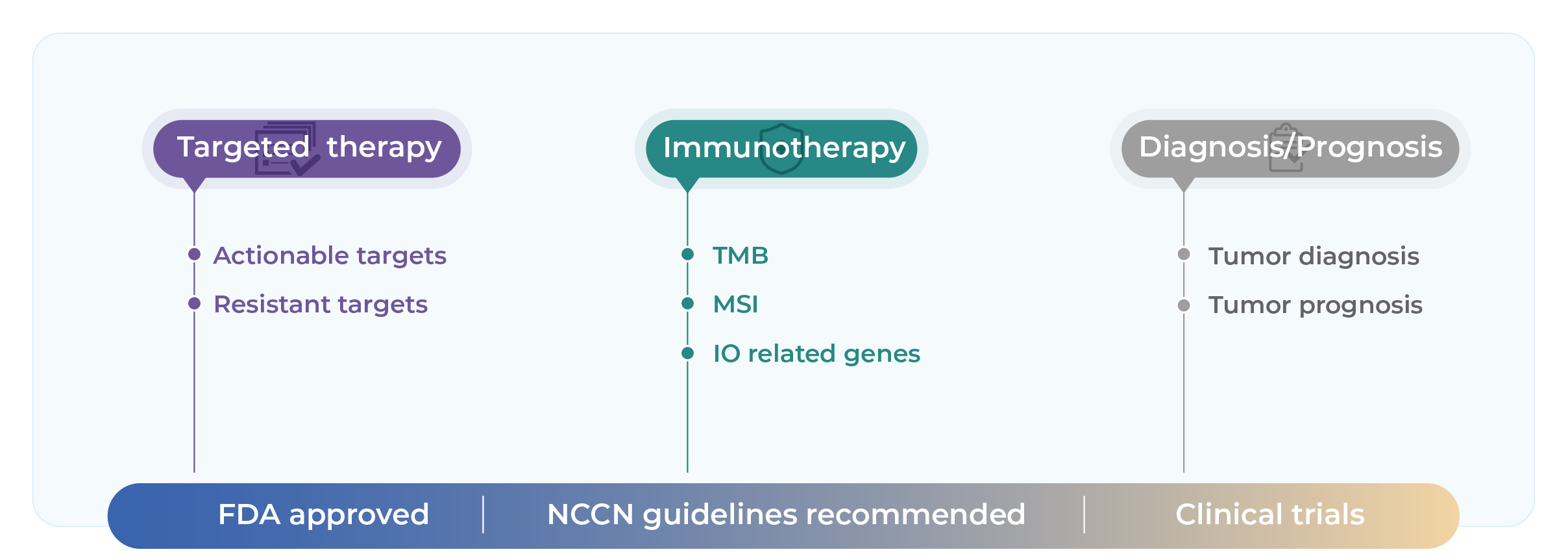
Genecast Comprehensive is suitable for:
Patients with common solid tumors
Patients requiring adjuvant or systemic therapy decision-making
Candidates for immunotherapy
Patients with existing or anticipated drug resistance
Patients seeking evaluation for clinical trial eligibility
Why Choose Genecast Comprehensive?
High-Precision Testing with Comprehensive Genomic Coverage
Covers 769 DNA genes and 52 fusion targets from FFPE tissue, enabling accurate identification of clinically actionable mutations across major solid tumors such as NSCLC and CRC.
End-to-End Clinical Support
From specimen logistics to expert report interpretation, Genecast provides full-service solutions designed to ease clinical workflow and empower evidence-based treatment decisions.
Proprietary Algorithms for Clinically Relevant Insights
Powered by the Genecast Smart-Reporter platform, our in-house algorithms integrate large-scale genomic datasets and curated clinical evidence to deliver meaningful and actionable results.
Clinician-Friendly Reporting
Structured and easy-to-navigate reports highlight drug-mutation associations, clinical trial opportunities, and levels of evidence—designed for fast interpretation and immediate application.
Trusted Oncology Partner
With over a decade of exclusive focus on solid tumor NGS, Genecast has become a trusted partner for leading cancer centers and physicians across the region.
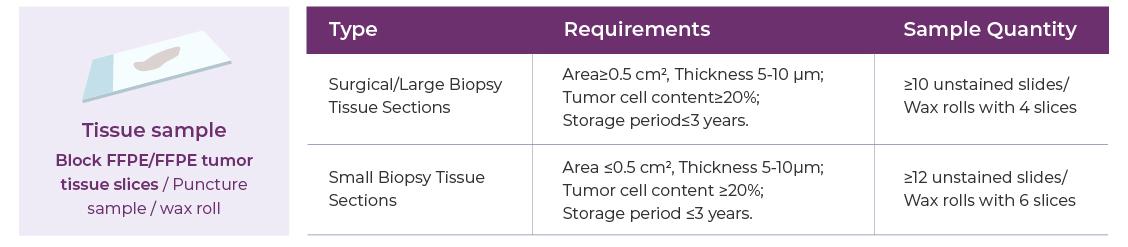
Sample Requirements
Testing Workflow & Ordering Process
TAT time:4-5 bussiness days*
[(*) Turn-around time takes effect upon sample arrival at Genecast Singapore lab and clears sample quality checks.]
Complete the Test Requisition Form
The ordering physician or patient fills out the form with relevant clinical and sample information.
Send Samples
Submit the required samples—such as FFPE blocks/slides/wax roll—to the Genecast laboratory.
Sample Receipt & Registration
Samples are inspected upon arrival and logged into our system for processing.
DNA & RNA Extraction
High-quality DNA and RNA are extracted from the submitted samples.
Library Preparation
Sequencing libraries are prepared using standardized protocols to ensure accuracy and consistency.
Next-Generation Sequencing
High-throughput sequencing is performed to detect relevant genomic alterations.
Bioinformatics Analysis
Advanced proprietary algorithms are applied to identify clinically actionable variants.
Report Delivery
The test report is generated and delivered to the designated email for clinical use.